Details of the Drug
General Information of Drug (ID: DM3NC4M)
| Drug Name |
Quercetin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Kvercetin; Meletin; QUE; Quercetine; Quercetol; Quercitin; Quertin; Quertine; Sophoretin; Xanthaurine; Flavin meletin; Kvercetin [Czech]; Quercetin content; Quercetin dihydrate; CI Natural Yellow 10; Cyanidelonon 1522; KUC104418N; KUC107684N; MixCom3_000183; Natural Yellow 10; P0042; Q 0125; TNP00070; TNP00089; LIM-5662; LNS-5662; C.I . natural yellow 10; C.I. 75670; C.I. Natural Yellow 10; C.I. Natural red 1; CU-01000012502-3; KSC-10-126; KSC-23-76; T-Gelb bzw. grun 1; C.I. Natural yellow 10 & 13; 2-(3,4-Dihydroxy-phenyl)-3,5,7-trihydroxy-chromen-4-one; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-1-benzopyran-4-one dihydrate; 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one; 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one; 3',4',5,7-Tetrahydroxyflavan-3-ol; 3',4',5,7-tetrahydroxyflavon-3-ol; 3,3',4',5,7-Pentahydroxyflavone dihydrate; 3,5,7,3',4'-Pentahydroxyflavone; 3,5,7-Trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-on; 3,5,7-trihydroxy-2-(3,4-dihydroxyphenyl)-4H-chromen-4-one
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Therapeutic Class |
Hypoglycemic Agents
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
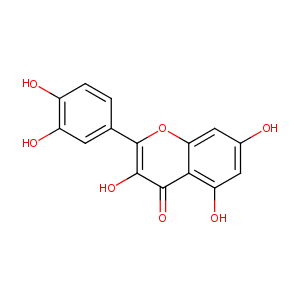 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 302.23 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 1.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 5 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Transporter (DTP) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
References
